If you find these notes really helpful and easy to understand. Do like, comment and share. Keep following Gone Nuclear for more notes on CBSE IX, X, XI & XII. Follow us on Facebook on Gone Nuclear
ELECTROCHEMISTRY
1. ELECTROCHEMISTRY
Electrochemistry is the study of production of electricity from the energy released during a spontaneous chemical reaction and the use of electrical energy to bring about non-spontaneous chemical transformations.
2. ELECTROCHEMICAL CELLS
A spontaneous chemical process is the one which can take place on its own and in such a process the Gibb's energy of the system decreases. It is this energy that gets converted to electrical energy. The reverse process is also possible in which we can make non-spontaneous processes occur by supplying external energy in the form of electrical energy. These inter conversations are carried out in equipment called electrochemical cells.
3. TYPES OF ELECTROCHEMICAL CELLS
Electrochemical cells are of two types.
3.1 Galvanic Cells - Converts chemical energy into electrical energy.
3.2 Electrolytic Cells - Converts electrical energy into chemical energy.
4. GALVANIC CELL
Cell energy is extracted from a spontaneous chemical process or reaction and it is converted electric current.
For example, Daniel Cell is a Galvanic Cell in which Zinc and Copper are used for the redox reaction to take place.
Zn(s) + Cu2+(aq) -> Zn2+(aq) + Cu(s)
Oxidation Half: Zn(s)-> Zn2+(aq) + 2e-
Reduction Half: Cu2+(aq) + 2e- -> Cu(s)
Zn is the reducing agent and Cu2+ acts as oxidising agent. The half cells are also known as electrodes.
The oxidation half cell is called anode and the reduction half cell is called cathode. Electrons flow from anode to cathode in the external circuit.
Anode is assigned negative polarity and cathode is assigned positive polarity.
In Daniel cell Zn acts as anode and Cu acts as cathode.

5. ELECTROLYTIC CELL
These electrodes are dipped in and electrolytic solutions containing cations and anions. On supplying current the ions move towards electrodes of opposite polarity and simultaneous reduction and oxidation processes takes place.

5.1 Preferential Discharge of Ions
Where there are more than one cation or anion the process of discharge becomes competitive in nature. Discharge of ions requires energy and in case of several ions being present the discharge of that ion will take place first which requires the energy.
6. ELECTRODE POTENTIAL
It may be defined as the tendency of an element, when it is placed in contact with its own ions to either lose or gain electrons and in turn becomes positively or negatively charged.
The electrode potential will be named as oxidation or reduction potential depending upon whether oxidation or reduction has taken place.
a) Both oxidation and reduction potential are equal in magnitude but opposite in sign.
b) It is not a thermodynamic property, so values of E are not additive.
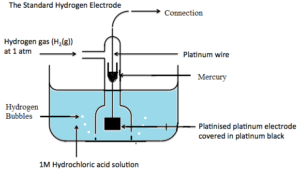
7. STANDARD ELECTRODE POTENTIAL (E0)
It may be defined as the electrode potential of an electrode determined relative of Standard Hydrogen Electrode (SHE) under standard conditions. The standard condition taken are:
i) 1M concentration of each ion solution.
ii) A temperature of 298 K.
iii) 1 bar pressure for each gas.
8. ELECTROCHEMICAL SERIES
The half cell potential values are standard values and are represented as the standard reduction potential values as shown in the table below which is also called electrochemical series.

9. CELL POENTIAL OR EMF OF A CELL
The difference between the electrode potentials of two half cells is called cell potential.
It is known as Electromotice force (EMF) of the cell if no current is drawn from the cell.
Ecell = Ecathode + Eanode
Ecell = Ereduction + Eoxidation
Ecell = Eright + Eleft
So for Daniel Cell,
10. CELL DIAGRAM OR REPRESENTATION OF A CELL
The following conventions or notations are applied for writing the cell diagram in accordance with IUPAC recommendations.
The Daniel Cell is represented as follows:
a) Anode half cell is written on the left hand side while cathode half cell is written in the right hand side.
b) A single vertical line (|) separates the metal from aqueous solution of its own ions.
c) A double vertical line (||) represents salt bridge.
d) The molar concentration (C) is placed in brackets () after the formula of the corresponding ion.
e) The value of EMF of the cell is written on the extreme right of the cell.
f) If an inert electrode like platinum is involved in the construction of the cell, it may be written along with the working electrode in brackets say for example, when a Zn anode is connected to a H2 electrode.
Salt bridge is used to maintain the charge balance and to complete the circuit by facilitating the flow of ions through it.
It contains a gel in which inert electrolyte like Na2SO4 or KNO3 etc are mixed with agar agar.
Negative ions flow to the anode and positive ions flow to the cathode through the salt bridge and charge balance is maintained and cell is kept on functioning.

12. SPONTANEITY OF A REACTION
If we take standard value of cell potential in the above equation we will obtain standard value for ΔG as well.
13. TYPES OF ELECTRODES
13.1 Metal-Metal Ion Electrodes
A metal rod/plate is dipped in an electrolytic solution containing metal ions. There is a potential difference between these two phases and this electrode can act as a cathode or anode both.
13.2 Gas Electrodes
Electrode gases like H2, Cl2 etc are used with their respective ions. For example, H2 gas is used with a dilute solution of HCl(H+ ions). The metal should be inert so that it does not react with the acid.
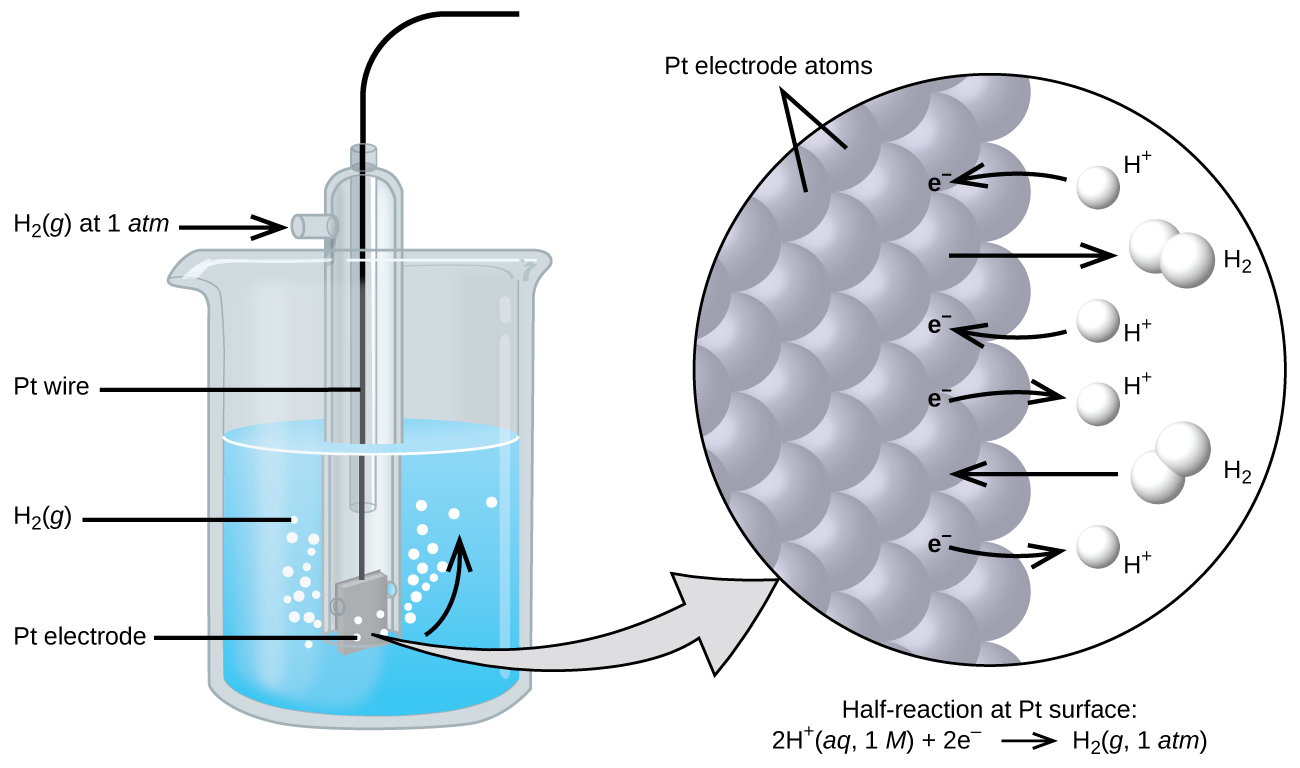
The hydrogen electrode is also used as the standard to measure other electrode potentials. Its own potential is set to 0 V as a reference. When it is used as a reference the concentration of dil. HCl is taken as 1 M and the the electrode is called Standard Hydrogen Electrode.
13.3 Metal-Insoluble Salt Electrodes
We use some metals which are sparingly soluble with the metal itself as electrodes. For example, if we use AgCl with Ag there is a potential gap between these two phases which can be identified in the following reaction.
13.4 Calomel Electrode
Mercury is used with two other phases, one is a calomel paste (Hg2Cl2) and electrolyte containing Cl- ions.
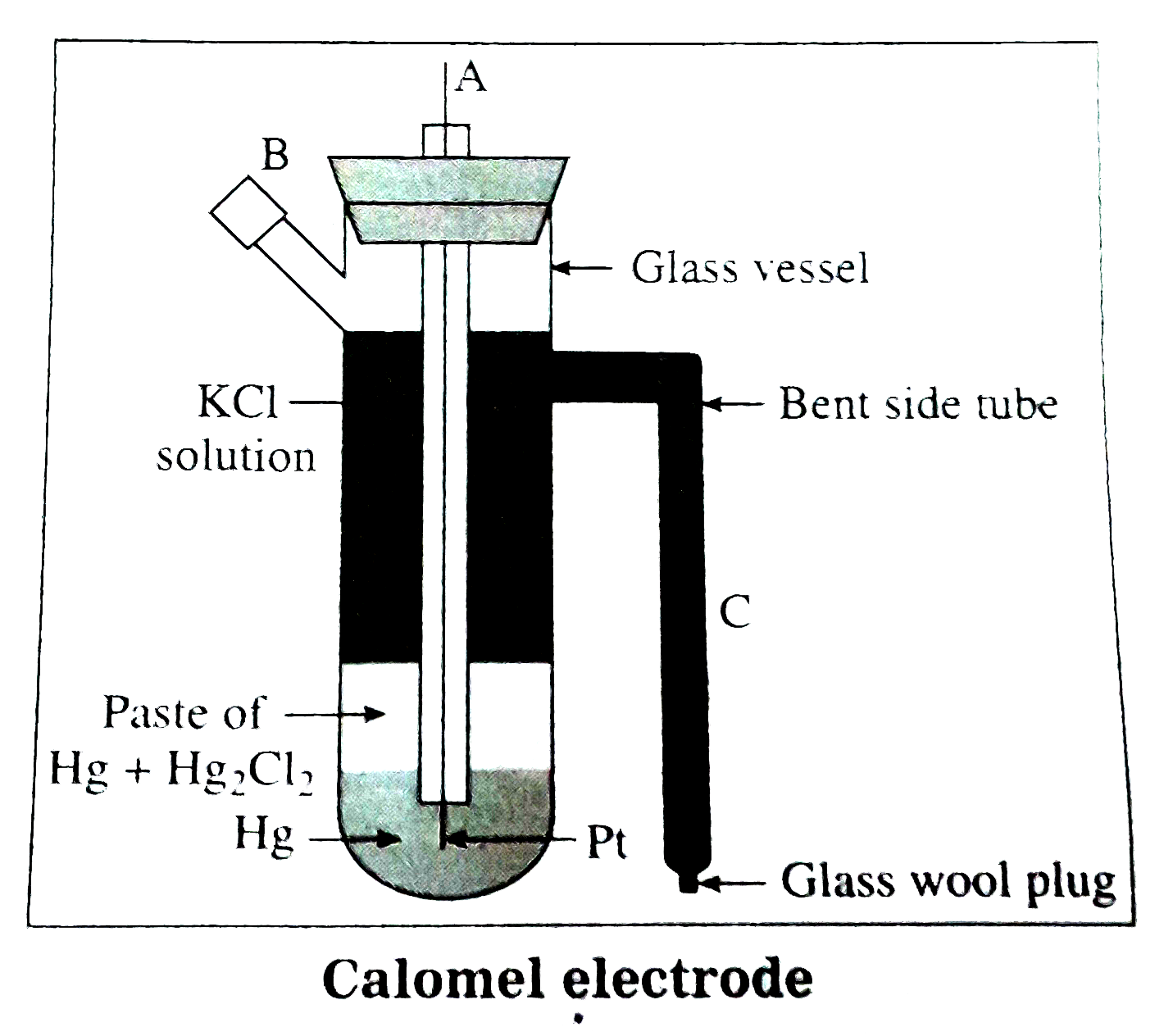
This electrode is also used as another standard to measure other potentials. Its standard form is also called Standard Calomel Electrode (SCE).
13.5 Redox Electrode
In these electrodes two different oxidation states of the same metal are used in the same half cell. For example, Fe2+ and Fe3+ are dissolved in the same container and an inert electrode of platinum is used for the electron transfer. Following reaction can take place.
14. NERNST EQUATION
It relates electrode potential with the concentration of ions.
Thus, the reduction potential increases with the increase in the concentration of ions.
For a general electrochemical reaction of the type.
15. APPLICATION OF NERNST EQUATION
15.1 Equilibrium Constant from Nernst Equation
For a Daniel cell at equilibrium,
16. CONCENTRATION CELLS
If the two electrodes of the same metal are dipped separately into two solutions of the same electrolytes having different concentrations and the solutions are connected through salt bridge, such calls are known as concentration cells. For example,
16.1 Electrode Concentration Cells
16.2 Electrolyte Concentration Cells
17. CASES OF ELECTROLYSIS
17.1 Electrolysis of molten Sodium Chloride
The reactions occurring at the two electrodes may be show as follows
17.2 Electrolysis of an aqueous solution of Sodium Chloride
Thus, H2 gas is evolved at cathode and Na+ ions remain in solution.
Thus, Cl2 gas is evolved at the anode by over voltage concept while OH- ions remain in the solution.
Thus, Cl2 gas is evolved at the anode by over voltage concept while OH- ions remain in the solution.
18. BATTERIES
When Galvanic Cells are connected in series to obtain a higher voltage, the arrangement is called Battery.
18.1 Primary Battery
Primary cells are those which can be used so long that the active minerals are present. Once they get consumed the cell will stop functioning and cannot be re-used. Example, Dry Cell, Leclanche cell and Mercury cell.
18.2 Dry Cell
The standard potential of this cell is 1.5 V and it falls as the cell gets discharged continuously and once used, it cannot be charged.
18.3 Mercury Cell
These are used in small equipment like watches, hearing aids.
18.4 Secondary Battery
Secondary cells are those which can be recharged again and again for multiple uses.
For example, Lead Storage Battery and Nickel Cadmium Battery.
18.5 Lead Storage Battery
To recharge the cell, it is connected with a cell of higher potential and this cell behaves as an electrolytic cell and the reactions are reversed. Pb(s) and PbO2(s) are regenerated at the respective electrodes. These cells deliver an almost consistent voltage.
19. FUEL CELL
A fuel cell differs from an ordinary battery in the sense that the reactants are not contained inside the cell but are externally supplied from an external reservoir.
Fuel cell is used in space shuttles and in this cell the two gasses are supplied from external storage.
In this cell carbon rods are used as electrodes with KOH as the electrolyte.

20. CORROSION
It involves a redox reaction and formation of an electrochemical cell on the surface of iron or other metals.
At one location oxidation of iron takes place (anode) and at another location reduction of oxygen to form water takes place (cathode).
First Fe gets oxidised to Fe2+ and then in the presence of oxygen it forms Fe3+ which then reacts with water to form rust which is represented by Fe2O3.xH2O.
Rusting of iron can be avoided by painting it or by coating it with some other metals like Zinc.
The later process is called galvanisation.
As the tendency of Zn to get oxidised is more than iron it gets oxidised in preference and iron is protected. This method of protecting one metal by the other is also called Cathodic Protection.
21. CONDUCTANCE (G)
It is the reciprocal of resistance and may be defined as the ease with which the electric current flows through a conductor.
22. CONDUCTIVITY (κ)
It is the reciprocal of resistivity (ρ).
Hence, conductivity of an electrolytic solution may be defined as the conductance of a solution of 1 cm length with area of cross-section equals to 1 cm^2.
23. FACTORS AFFECTING ELECTROLYTIC CONDUCTANCE
23.1 Electrolyte
An electrolyte is a substance that dissociates in solution to produce ions and hence conducts electricity in dissolved or molten state.
For example, HCl, NaOH, KCl are strong electrolytes and CH3COOH, NH4OH are weak electrolytes.
The conductance of electricity by ions present in the solution is called electrolytic or ionic conductance. The following factors govern the flow of electricity through a solution of electrolyte.
a) Nature of electrolyte or inter-ionic attractions- Lesser the solute-solute interactions, greater will be the freedom of movement of ions and higher will be the conductance.
b) Solvation of ions- Larger the magnitude of solute-solvent interactions, greater is the extent of solvation and lower will be the electrical conductance.
c) Nature of solvent and its viscosity- Larger the solvent-solvent interaction, larger will be viscosity and more will be the resistance offered by the solvent to flow of ions hence lesser will be the electrical conductance.
d) Temperature- As the temperature of electrolytic solution rises solute-solute, solute-solvent and solvent -solvent interaction decreases, this leads to the increase of electrical conductance.
24. MEASUREMENT OF CONDUCTANCE
As we know, κ = 1/R x l/A
The value of κ could be known if we measure l, A and R.
The value of resistance of the solution R between two parallel electrodes is determined by using Wheatstone Bridge method.
It consists of two fixed resistances R3 and R4, a variable resistance R1 and the conductivity cell having the unknown resistance R2. The bridge is balanced when no current is passes through the detector. Under these conditions,
25. MOLAR CONDUCTIVITY (Λm)
It may be defined as the conducting power of all the ions produced by dissolving one mole of any electrolyte placed between two large electrodes at one centimetre apart.
Mathematically,
where, V is the volume of solution in cm^3 containing 1 mole of electrolyte and C is the molar concentration.
26. EQUIVALENT CONDUCTIVITY (Λeq)
It is conducting power of one equivalent of electrolyte placed between two large electrodes at one centimetre apart.
Mathematically,
Where, V is the volume of solution in cm^3 containing 1 equivalent of electrolyte and N is normality.
27. VARIATION OF CONDUCTIVITY AND MOLAR CONDUCTIVITY WITH DILUTION
Conductivity decreases with decrease in concentration, this is because the number of ions per unit volume that carry the current in the solution decreases on dilution.
Molar conductivity increases with decrease in concentration. This is because the total volume of solution containing one mole of electrolyte also increases.
It has been found that the decrease in κ on dilution of a solution is more than compensated by increase in its volume.
28. LIMITING MOLAR CONDUCTIVITY (Λm)
The value of molar conductivity when the concentration approaches zero is known as limiting molar conductivity at infinite dilution.
It is possible to determine the molar conductivity at infinite dilution (Λ0m) in case of strong electrolytes by extrapolation of curve Λm vs √C. On contrary, the value of molar conductivity of weak electrolyte at infinite dilution cannot be determined by extrapolation of the curve as the curve becomes almost parallel to y-axis when concentration approaches to zero.
The mathematical representation between Λm and Λ0m for strong electrolyte was developed by Debye, Huckel and Onsagar.
In simplified form the equation can be given as
29. KOHLRAUSCH'S LAW
It states that the limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
In general, if an electrolyte on dissociation given V+ cations and V_ anions then its limiting molar conductivity is given by
Here, λ0+ and λ0- are the limiting molar conductivities of cations and anions respectively.
30. APPLICATIONS OF KOHLRAUSCH'S LAW
30.1 Calculation of molar conductivities of weak electrolytes at infinite dilution
For example, molar conductivity of acetic acid at infinite dilution can be obtained from the knowledge of molar conductivity at infinite dilution of strong electrolytes like HCl, CH3COONa and NaCl as shown below.
30.2 Determination of Degree of Dissociation of Weak Electrolytes
30.3 Determination of Dissociation Constant (K) of Weak Electrolytes
31. USE OF ΔG IN RELATING EMF VALUES OF HALF CELL REACTIONS
When we have two half cells reactions such that on adding them we obtain another half cell reaction then their EMFs cannot be added directly.
But in any case thermodynamic functions like ΔG can be added and EMF values can be related through them.
Consider the following three half cell reactions,
We can easily observe that the third reaction can be obtained by subtracting the first reaction from the second.
But the same relation does not apply on the EMF values. That is E3≠E2-E1. But the ΔG values can be related according to the relations. That is,
Note- We should always remember that EMF values are additive only when two half cell reactions are added to give a complete balanced cell reaction. In any other case we will be using ΔG values to obtain relation between EMF values.
---X---
If you find these notes really helpful and easy to understand. Do like, comment and share. Keep following Gone Nuclear for more notes on CBSE IX, X, XI & XII. Follow us on Facebook on Gone Nuclear

Atish J. Bain
HOD (Dept. of Chemistry)
St. Thomas High School (10+2)
Dhanbad, Jharkhand





































































No comments:
Post a Comment
Thank you. Will get back to you.