If you find these notes helpful and easy to understand, do like, comment and share. Keep following us on Facebook "Gone Nuclear". For more notes and videos on CBSE IX, X, XI and XII Chemistry. Keep following us here on blogger "Gone Nuclear".
Matter In Our Surroundings
Particle Nature of Matter
Anything that occupies space and has mass and is felt by senses is called matter.
The matter is the form of five basic elements the Panch tatva – air, earth, fire, sky and water.
Characteristics of particles of matter
Made of tiny particles.
Vacant spaces exist in particles.
Particles are in continuous motion.
Particles are held together by forces of attraction.
Q.1 Define matter.
Q.2 What happens if you put copper sulphate crystals in the water?
States of Matter
Basis of Classification of Types
Based upon particle arrangement
Based upon the energy of particles
Based upon distance between particles


Q.1 A substance has a definite volume but no definite shape? State whether this substance is a solid, a liquid or a gas.
Q.2 Arrange the following substances in increasing order of force of attraction between the particles. (a) Milk (b) Salt (c) Oxygen.
Q.3 A substance has neither a fixed shape nor a fixed volume. State whether it is a solid , a liquid or a gas.
Q.4 The melting point of a substance is below the room temperature. Predict its physical state.
Interchange in states of matter
Matter Can Change its State
Water can exist in three states of matter –
• Solid, as ice,
• Liquid, as the familiar water, and
• Gas, as water vapour.
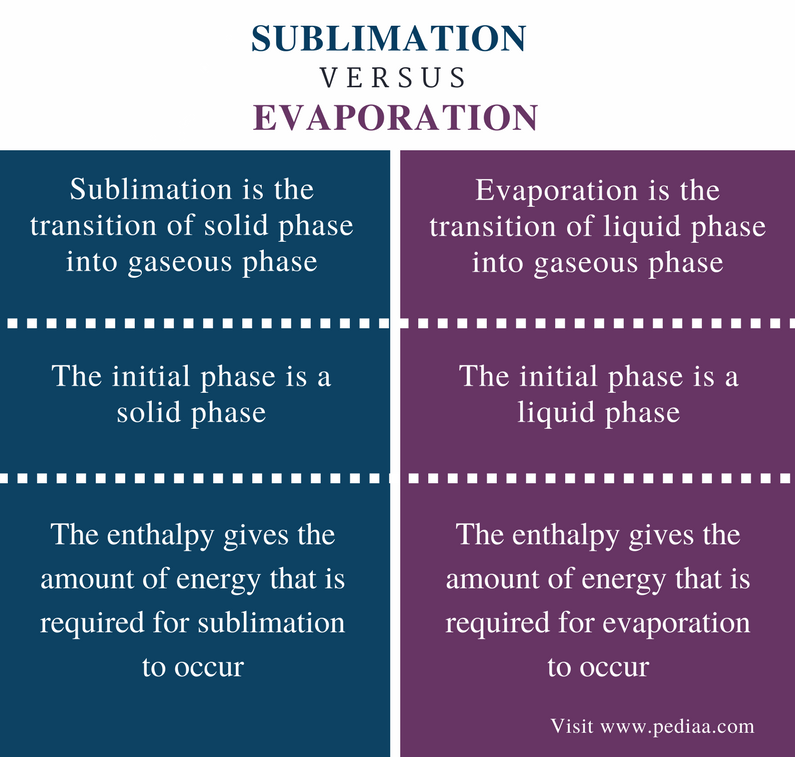
Sublimation
The changing of solid directly into vapours on heating & vapours into solid on cooling. Ex. Ammonium chloride, camphor & iodine.
a) Effect of change in temperature
The temperature effect on heating a solid varies depending on the nature of the solid & the conditions required in bringing the change.
On increasing the temperature of solids, the kinetic energy of the particles increases which overcomes the forces of attraction between the particles thereby solid melts and is converted to a liquid.
The temperature at which solid melts to become a liquid at the atmospheric pressure is called its melting point.
The melting point of ice is 273.16 K.
The process of melting, that is, change of solid state into a liquid state is also known as fusion.
b) Effect of Change of Pressure
Increasing or decreasing the pressure can change the state of matter. Applying pressure and reducing temperature can liquefy gases.
Solid carbon dioxide (CO2) is stored under high pressure. Solid CO2 gets converted directly to gaseous state on a decrease of pressure to 1 atmosphere without coming into the liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
Latent Heat
The hidden heat which breaks the force of attraction between the molecules during the change of state.


We can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
Q.1 What is vapour?
Q.2 Name the temperature at which the solid and liquid states of substance can exist together.
Q.3 What is the effect of pressure on boiling point?
Q.4 Name any two substances which sublime.
Q.5 Define Condensation.
Q.6 For any substance, why does the temperature remain constant during the change of state?
Evaporation & Boiling
Particles of matter are always moving and are never at rest.
At a given temperature in any gas, liquid or solid, there are particles with different amounts of kinetic energy.
In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour.
This phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
Factors Affecting Evaporation
The rate of evaporation increases with an increase in surface area.
With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
Humidity is the amount of water vapour present in the air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the amount of water in the air is already high, the rate of evaporation decreases.
Wind speed: the higher the wind speed, the more evaporation.
Evaporation cause cooling.
The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation.
Evaporation Vs Boiling
Boiling is a bulk phenomenon. Particles from the bulk (whole) of the liquid change into the vapour state.
Evaporation is a surface phenomenon. Particles from the surface gain enough energy to overcome the forces of attraction present in the liquid and change into the vapour state.
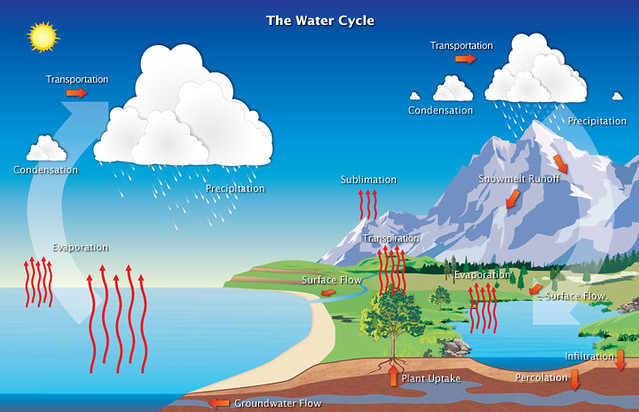
Let us look at the water cycle and relate.
Q.1 Which is the slow process, Evaporation or Boiling?
Q.2 State the effect of surface area on the rate of evaporation.
Q.3 Why are we able to sip hot tea faster from saucer rather than from a cup?
Kelvin & Celsius Scale
Kelvin is the SI unit of temperature, 0 C =273.16 K. we take 0 C = 273 K.
SI unit of temperature is Kelvin. T (K)= T (C) +273
Kelvin scale of temperature has always a positive sign, hence regarded as better scale than Celsius.
Atmosphere (atm) is a unit of measuring the pressure exerted by a gas. The SI unit of pressure is Pascal (Pa):
1 atmosphere = 1.01 × (10^5) Pa. The pressure of air in the atmosphere is called atmospheric pressure. The atmospheric pressure at sea level is 1 atmosphere and is taken as the normal atmospheric pressure.
Q.1 What is the SI unit of temperature?
Q.2 Kelvin scale of temperature is regarded as a better scale than Celsius. Why?
Q.3 Convert 10oC into Kelvin scale.
QUESTION BANK
1 Mark Questions:
1. Pressure on the surface of a gas is increased. What will happen to the interparticle forces?
2. Name the three states of matter.
3. What happens when a liquid is heated?
4. A gas can exert pressure on the walls of the container. Assign reason.
5. Convert the following temperature to Kelvin Scale (a) 100°C (b) 37°C
6. What is meant by density?
7. Give the characteristics of the particles of matter.
8. Water droplets seen on the outer surface of a glass containing ice-cold water is due to _____________.
9. Change of gaseous state directly to solid state without going through liquid state is called _____________________ .
10. __________________ is a surface phenomenon.
2 Marks Questions:
1. Define Latent heat of vaporisation.
2. Explain why temperature remains constant during the change of state of any substance?
3. Define Sublimation with examples.
4. *Do we sweat more on a dry day or humid day? Justify your reason.
5. Why do we see water droplets on the outer surface of a glass containing ice-cold water?
6. Convert the following temperature to the Kelvin scale (a) 25°C (b) 373°C
7. List two properties that liquids have in common with solids.
8. List two properties that liquids have in common with gases.
9. What will happen to the melting point temperature of ice if some common salt is added to it? Justify your answer.
10. How will you show that air has a maximum compressibility?
3 Marks Questions:
1. Define the term (a) Latent heat of fusion (b) Latent heat of vaporization
2. State the effect of (i) surface area (ii) nature of the liquid on the rate of evaporation.
3. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Why?
4. What is the physical state of water at 250°C, 100°C, 0°C?
5. Give reasons :
i) A sponge can be pressed easily; still, it is called a solid.
ii) Water vapours have more energy than water at the same temperature.
6. What are the intermolecular forces? How are these related to the three states of matter?
7. Is it possible to liquefy atmospheric gases? If yes, suggest a method.
5 marks Questions:
1. a) What is meant by evaporation? What are the factors on which the rate of evaporation depends upon?
b) How does evaporation causes cooling?
2. State the properties of all the five states of matter.
3. Define: Melting point, Freezing point & Boiling point
If you find these notes helpful and easy to understand, do like, comment and share. Keep following us on Facebook "Gone Nuclear". For more notes and videos on CBSE IX, X, XI and XII Chemistry. Keep following us here on blogger "Gone Nuclear".

Atish Bain
HOD (Dept. of Chemistry)
St. Thomas High School (10+2)
Dhanbad, Jharkhand









No comments:
Post a Comment
Thank you. Will get back to you.